-
UBASTEC-AUTO
-
Early Detection of Biliary Atresia
Biliary atresia is one of intractable pediatric diseases said to occur in 1 out of 10,000 neonates. Its early detection and surgical treatment within 2 months after birth are necessary, and some methods for early detection have been tried; however, it continues to be detected occasionally after a duration of 2 months.
Currently, much attention has been paid to the hepatic function test with widdle as a method for early detection of biliary atresia, and enlightenment activities have been made actively.
-
Early Detection and Surgery within Two Months of Age
The pathway through which bile produced in the liver flows to the intestines are called the bile passage or the bile duct. If the bile passage or bile duct may become blocked at birth or shortly after birth for some reason and become unable to flow, then the bile may become stagnant in the liver and flow back into the bloodstream, causing jaundice and whitish stools.
If left untreated, hepatic function is gradually lost, and the liver gradually becomes hardened and cirrhotic, eventually leading to death. This disease is one of the intractable pediatric diseases and is said to occur in one out of every 10,000 children. In each year, about 100 babies are affected by this disease in Japan.
The prognosis is often poor when the disease is not detected early in life and surgery is not performed within two months of age. Even if the surgery is performed after two months and the bile begins to flow out of the liver, the disease has already progressed to cirrhosis and thus the patient will have sequelae.
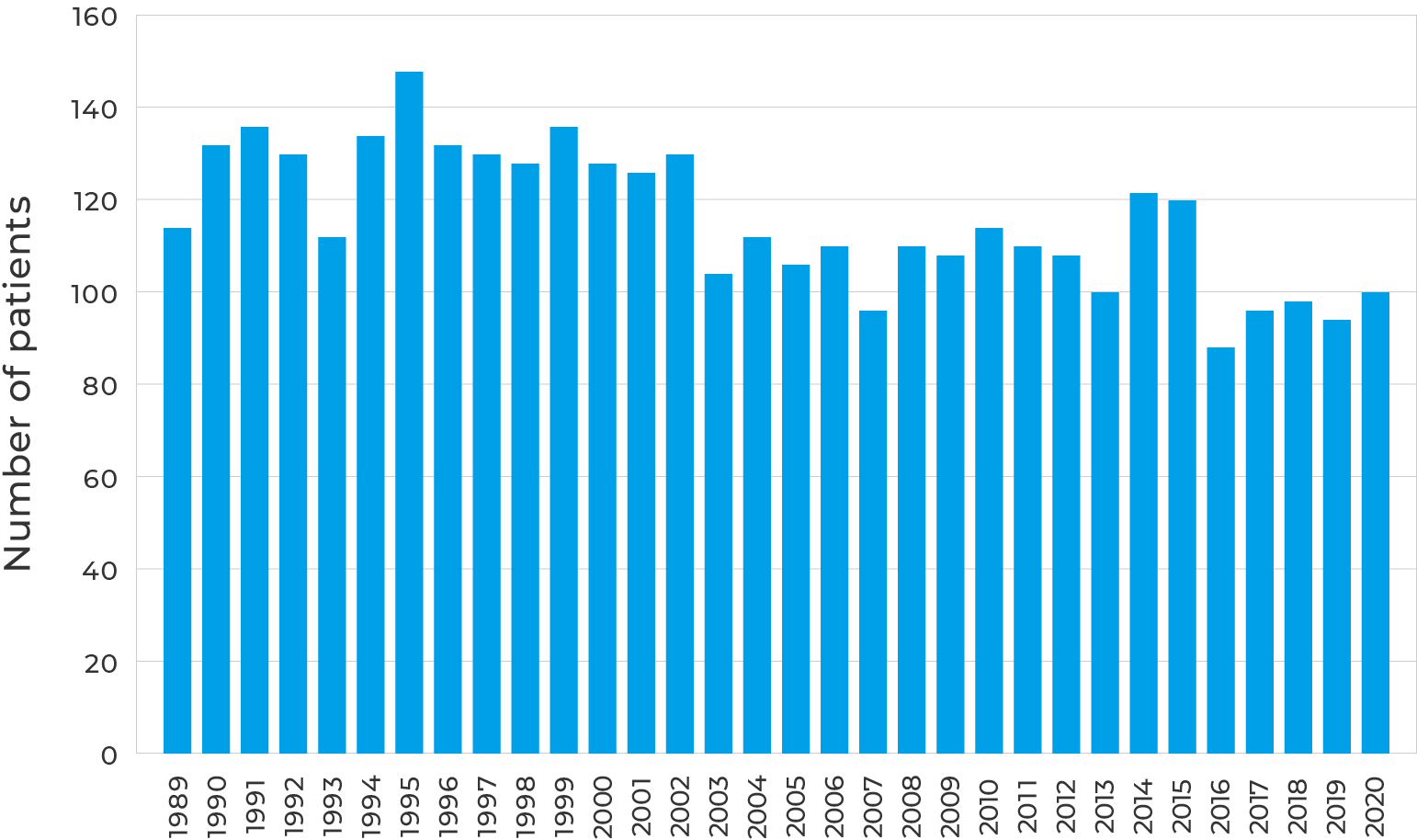
Number of registered cases of biliary atresia in Japan (from 1989 to 2020) (based on Japan Biliary Atresia Study Group and National Registry Office for Biliary Atresia, Journal of the Japan Society for Biliary Atresia, 58, 201 (2022))
-
Hepatic Function Test with Widdle
While blood samples are commonly used to test hepatic function, Sanyo Fine Co., Ltd. have developed a new method using “widdle”. This makes it possible to easily test hepatic function even in newborns, whose blood vessels are narrow and thus difficult to conduct blood samplings.
-
Automatic analyzer for laboratory examination
-
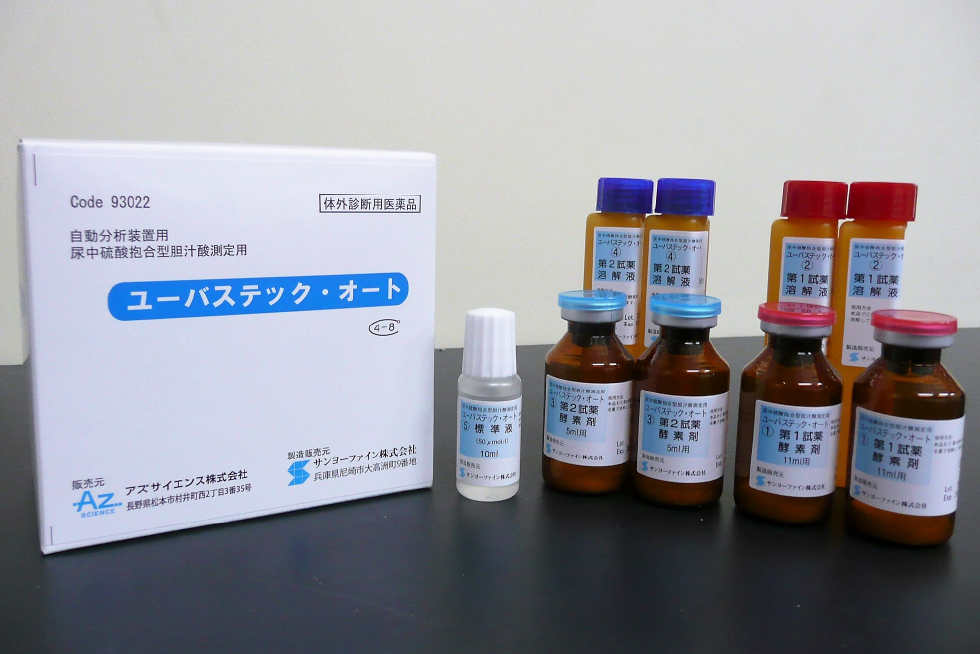
UBASTEC-AUTO,For the assay of urinary bile acid sulfates
-
Enlightenment Activities
The Association for Saving Children with Biliary Atresia, a family group of patients with biliary atresia, holds a forum on pediatric intractable diseases as an enlightenment activity on the importance of early detection and improvement of therapeutic methods to overcome biliary atresia.
Through the implementation of this forum, they are actively lobbying Japanese government and other related organizations to introduce USBA testing in infant health checkups.
-
The 7th forum: December 21, 2019
| Venue | Sendai International Center (Sendai, Miyagi, Japan) |
|---|---|
| Theme | Proposals for Early Detection and Diagnosis of Biliary Atresia Contents |
| Contents |
|
-
ARABINITEC-AUTO
-
Determination of D-Arabinitol in Serum
D-arabinitol is the characteristic a metabolite produced by genus Candida which is well known to cause a deepseated mycosis in mammals. The serum level of D-arabinitol is known to increase when Candida yeast proliferates in the body of a patient having an invasive candidiasis. Thus determination of serum level of D-arabinitol could be a good marker for the attenuation of a Candida species in a body.
ARABINITEC-AUTO is a simple assay reagent kit for the automatic measurement of D-arabinitol in serum. This kit utilizes an enzyme system which comprises D-arabinitol dehydrogenase combined with a color development system of diaphorase with WST-1, and enables a rapid and highly sensitive determination of D-arabinitol in a single channel using a self blank method without pretreatment.
-
UBASTEC-AUTO
-
In-vitro diagnostics
Reagent for determination of urinary bile acid sulfate (for automatic analyzers)
Certification Number: 222AHAMX00022000
-
A revolutionary test method that allows liver function testing without blood sampling.
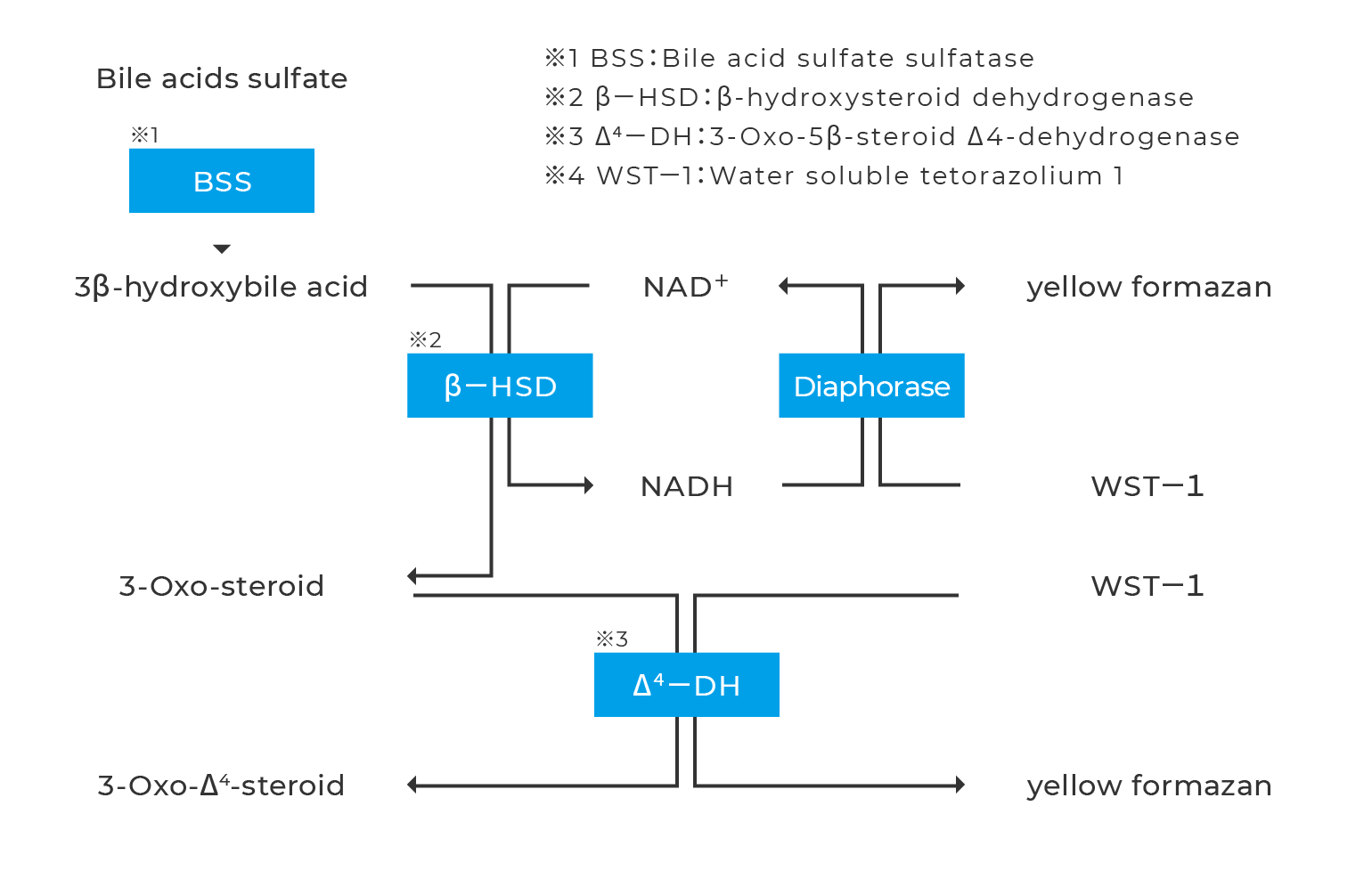
Assay Principal
Package
| Name | Constituent reagent | Package | ||
|---|---|---|---|---|
| UBASTEC-AUTO | Reagent 1 | Enzyme | For 11 ml-solvent | ×2 |
| Solvent | 11 ml | ×2 | ||
| Reagent 2 | Enzyme | For 5 ml-solvent | ×2 | |
| Solvent | 5 ml | ×2 | ||
| Standard solution | 10 ml | ×1 | ||
References
- *1 Uenoyama R., Baba S., et al : KAN.TAN.SUI (Japanese), 31, 315-326 (1995)
- *2 Meng G., Mashige F., Kitamura K., et al : Jpn. J. Clin. Chem., 23, 150-157 (1994)
- *3 Adachi K., Kakigawa K., et al : Jpn. J. Clin. Chem., 26, 95-100 (1998)
Distributed by AZ Science Co., Ltd.
-
ARABINITEC-AUTO
-
Reagent for determination of D-arabinitol in serum (for automatic analyzer)
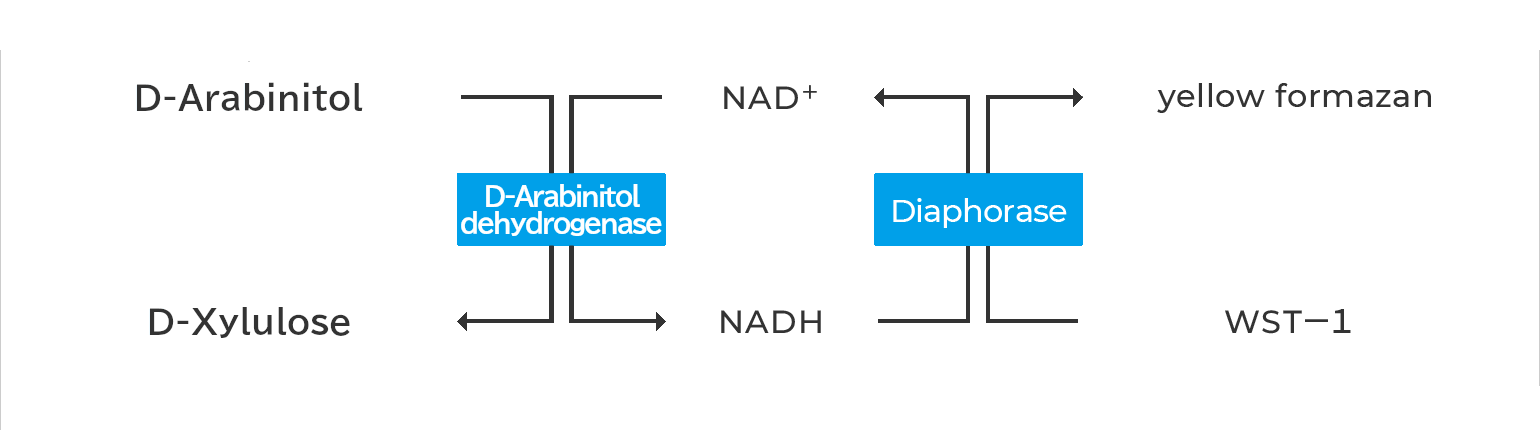
Assay Principal
Package
| Name | Constituent reagent | Package | ||
|---|---|---|---|---|
| ARABINITEC-AUTO | Reagent 1 | Enzyme | For 16 ml-solvent | ×4 |
| Solvent | 16 ml | ×4 | ||
| Reagent 2 | Enzyme | For 5 ml-solvent | ×4 | |
| Solvent | 5 ml | ×4 | ||
| Standard solution | 20 μM | 10 ml | ×1 | |
| 100 μM | 10 ml | ×1 | ||
| 1,000 μM | 10 ml | ×1 | ||