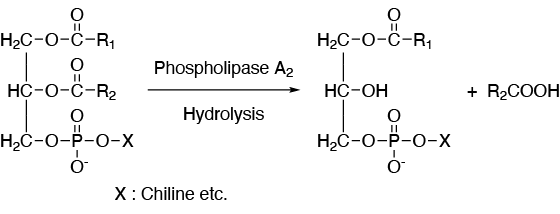-
Bio-Based Optical Resolution Technology
-
Contributing to Drug Development with Proprietary Biotechnologies
In order to meet the increasing needs for optically active compounds in recent years, we are focusing on research and development of optically active compounds utilizing stereoselectivity of microorganisms and enzymes.
We are also developing production technologies for alcohols, carboxylic esters, and their derivatives with high optical purity using our proprietary biotechnologies such as capitalization and enzymatic methods.
-
Sialic Acid Business
-
The World’s Leading Supplier with Various Patents
Sialic acid, which exists at the ends of sugar chains on the cell surface, is a kind of acidic sugar that transmits information between cells and performs receptor-like functions. Naturally occurring sialic acid is found in the nests of sea swallows, milk, eggs, etc., but the amount of sialic acid produced from these sources is limited in terms of quantity and cost.
Sanyo Fine has succeeded in producing high quality sialic acid in large quantities at low cost using its proprietary technology. Today, sialic acid is attracting attention for its application in pharmaceuticals and reagents, such as for use in anti-influenza drugs.
-
Pharmacological Function ・Learning ability improvement effect
・Infection prevention effect
・Phlegm expectorant effect
・Therapeutic effect on amyotrophyApplications ・Research reagent
・Raw materials for pharmaceuticals
・Raw materials for diagnostic reagents
・Virus filters
・Virus sensors- Pharmacological Function
-
・Learning ability improvement effect
・Infection prevention effect
・Phlegm expectorant effect
・Therapeutic effect on amyotrophy - Applications
-
・Research reagent
・Raw materials for pharmaceuticals
・Raw materials for diagnostic reagents
・Virus filters
・Virus sensors
-
Formula 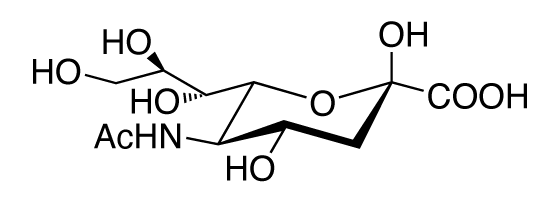
Sialic Acid (N-Acetylneuraminic Acid: NeuAc)
-
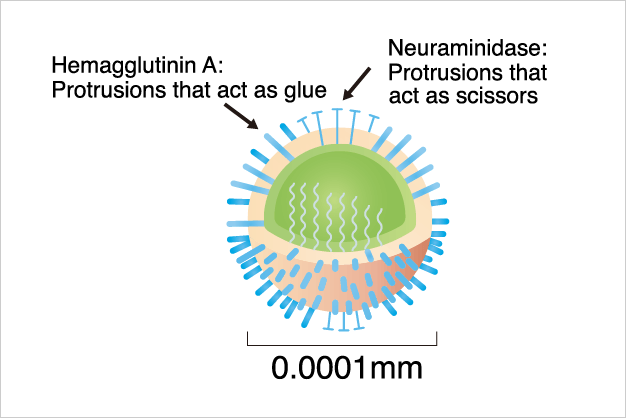
Sialic acid has been applied to medicines effective against influenza viruses.
Influenza viruses bind to and then invade the cells of the target of infection. They proliferate within the cells, and then exit the cell to infect other cells. The virus is shaped like a “chestnut” with numerous spike-like protrusions. There are two types of protrusions, which act as a “glue” and “scissors,” respectively.
First, the virus utilizes the “glue” protrusions to attach to sialic acid on the cell surface. Then it infiltrates into the cell and proliferats. Next, by use of the “scissors” protrusions, the proliferated viruses exfiltrate from the cell by breaking the bonds between them and the cell. The exfiltrated viruses invade other cells one after another with the same propagation mechanism.
An anti-influenza drug, made from our sialic acid, relieves flu symptoms by neutralizing the function of these “scissors. This means that viruses that have entered cells and multiplied cannot leave the cells, even if they multiply, because the “scissors” are neutralized by the drug. -
Carbohydrate Related Compounds
-
N-Acetylneuraminic acid [NANA; Sialic acid; NeuAc] [CAS RN: 131-48-6]
Prepared by enzymatic synthesis from N-acetylglucosamine and pyruvic acid.*1
References
- *1 I. Maru, J. Ohnishi, Y. Ohta and Y. Tsukada, Carbohydr. Res., 306, 575 (1998).
-
Formula 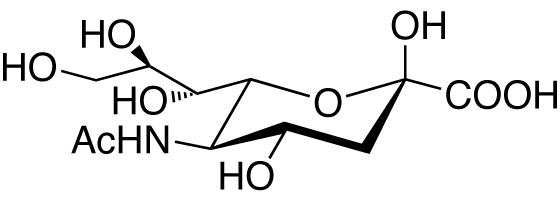
C11H19NO9 (MW: 309.27)
| CAS RN | 131-48-6 | Appearance | White crystalline powder |
|---|---|---|---|
| Purity | More than 99% (HPLC) | Package | 1 g, 10 g, 100 g, 500 g, Bulk |
- CAS RN
- 131-48-6
- Appearance
- White crystalline powder
- Purity
- More than 99% (HPLC)
- Package
- 1 g, 10 g, 100 g, 500 g, Bulk
-
N-Acetyl-D-mannosamine, monohydrate [ManNAc] [CAS RN: 7772-94-3]
N-Acetyl-D-mannosamine is an isomer of N-acetyl-D-glucosamine. It has attracted attention not only as an analytical standard but also for its use in the study of amino sugar metabolism and as an additive for animal cell culture. In recent years, there have been reports *1-*3 on the synthesis of sialic acid derivatives from derivatives of N-acetyl-D-mannosamine by condensation reaction with pyruvic acid using sialic acid aldolase.
References
- *1 M. J. Kim, W. J. Hennen, H. M. Sweers and C. H. Wong, J. Am. Chem. Soc., 110, 6481-6486 (1988).
- *2 C. Auge, B. Bouxom, B. Cavaye and C. Gautheron, Tetrahedron Lett., 30, 2217-2220 (1989).
- *3 C. C. Lin, C. H. Lin and C. H. Wong, Tetrahedron Lett., 38, 2649-2652 (1997).
-
Formula 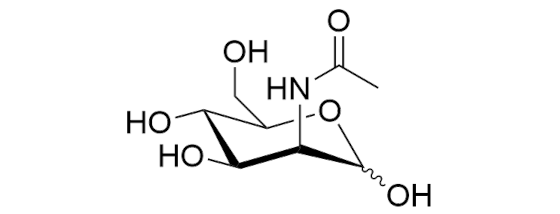
C8H15NO6・H2O (MW:239.2)
| CAS RN | 7772-94-3 | Appearance | White amorphous powder |
|---|---|---|---|
| Purity | More than 99% (HPLC) | Package | 1 g, 10 g, Bulk |
- CAS RN
- 7772-94-3
- Appearance
- White amorphous powder.
- Purity
- More than 99% (HPLC)
- Package
- 1 g, 10 g, Bulk
-
2,3-Dehydro-2-deoxy-N-acetylneuraminic acid [NeuAc2en; NeuNAc2en; Neu5Ac2en] [CAS RN: 24967-27-9]
Prepared by chemical synthesis from N-acetylneuraminic acid. Purified by column chromatography and crystalization.
-
Formula 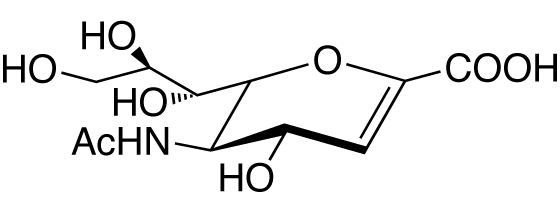
C11H17NO8 (MW: 291.25)
| CAS RN | 24967-27-9 | Appearance | White crystalline powder |
|---|---|---|---|
| Purity | More than 95% (HPLC) | Package | 25 mg, Bulk |
- CAS RN
- 24967-27-9
- Appearance
- White crystalline powder
- Purity
- More than 95% (HPLC)
- Package
- 25 mg, Bulk
-
N-Acetylneuraminic acid oligomer, sodium salt
Prepared by hydrolysis of colominic acid.
-
Formula n = 0 to 4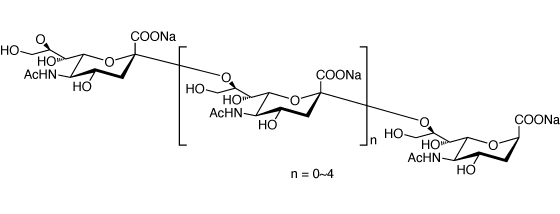
Dimer (DP2): C22H34N2O17Na2 (MW: 644.5)
Trimer (DP3): C33H50N3O25Na3 (MW: 957.73)
Tetramer (DP4): C44H66N4O33Na4 (MW: 1270.97)
Pentamer (DP5): C55H82N5O41Na5 (MW: 1584.21)
Hexamer (DP6): C66H98N6O49Na6 (MW: 1897.45)
| CAS RN | Appearance | White lyophilized powder | |
|---|---|---|---|
| Purity | More than 95% (HPLC) | Package |
DP2: 100 mg DP3 to DP6: 25 mg each |
- CAS RN
- Appearance
- White lyophilized powder
- Purity
- More than 95% (HPLC)
- Package
- DP2: 100 mg
DP3 to DP6: 25 mg each
-
Cytidine-5′-monophospho-N-acetylneuraminic acid, disodium salt [CMP-Neu5Ac·2Na] [CAS RN: 3063-71-6]
Prepared by enzymatic synthesis from N-acetylneuraminic acid and CTP. Purified by ion-exchange column chromatography and lyophilization.
-
Formula 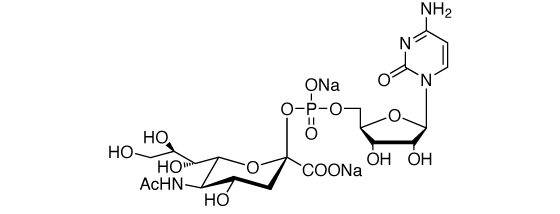
C20H29N4O16PNa2 (MW: 658)
| CAS RN | 3063-71-6 | Appearance | White lyophilized powder |
|---|---|---|---|
| Purity | More than 95% (HPLC) (Water content: 10% or less) | Package | 10 mg, 100 mg, 1 g, Bulk |
- CAS RN
- 3063-71-6
- Appearance
- White lyophilized powder
- Purity
- More than 95% (HPLC) (Water content: 10% or less)
- Package
- 10 mg, 100 mg, 1 g, Bulk
-
Uridine-5′-diphosphoglucose, disodium salt [CAS RN: 28053-08-9]
-
Formula 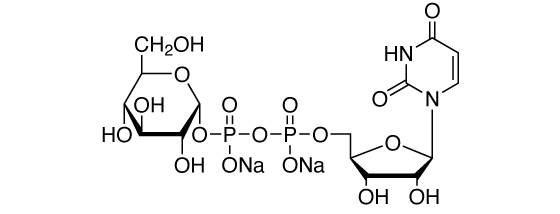
C15H22N2O17P2Na2 (MW: 610.3)
| CAS RN | 28053-08-9 | Appearance | White crystalline powder |
|---|---|---|---|
| Purity | More than 98% (Water content: approx. 7%) | Package | 1 g |
- CAS RN
- 28053-08-9
- Appearance
- White crystalline powder
- Purity
- More than 98% (Water content: approx. 7%)
- Package
- 1 g
-
Uridine-5′-diphosphoglucuronic acid, trisodium salt [CAS RN: 63700-19-6]
This product can be used as a substrate for glucuronidation for the purpose of pharmacokinetic studies.
-
Formula 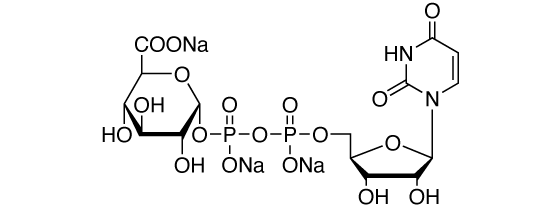
C15H19N2O18P2Na3 (MW: 646.3)
| CAS RN | 63700-19-6 | Appearance | White crystalline powder |
|---|---|---|---|
| Purity | More than 98% (Water content: 12% or less) | Package | 10 g, Bulk |
- CAS RN
- 63700-19-6
- Appearance
- White crystalline powder
- Purity
- More than 98% (Water content: 12% or less)
- Package
- 10 g, Bulk
Nucleic acid relatives
-
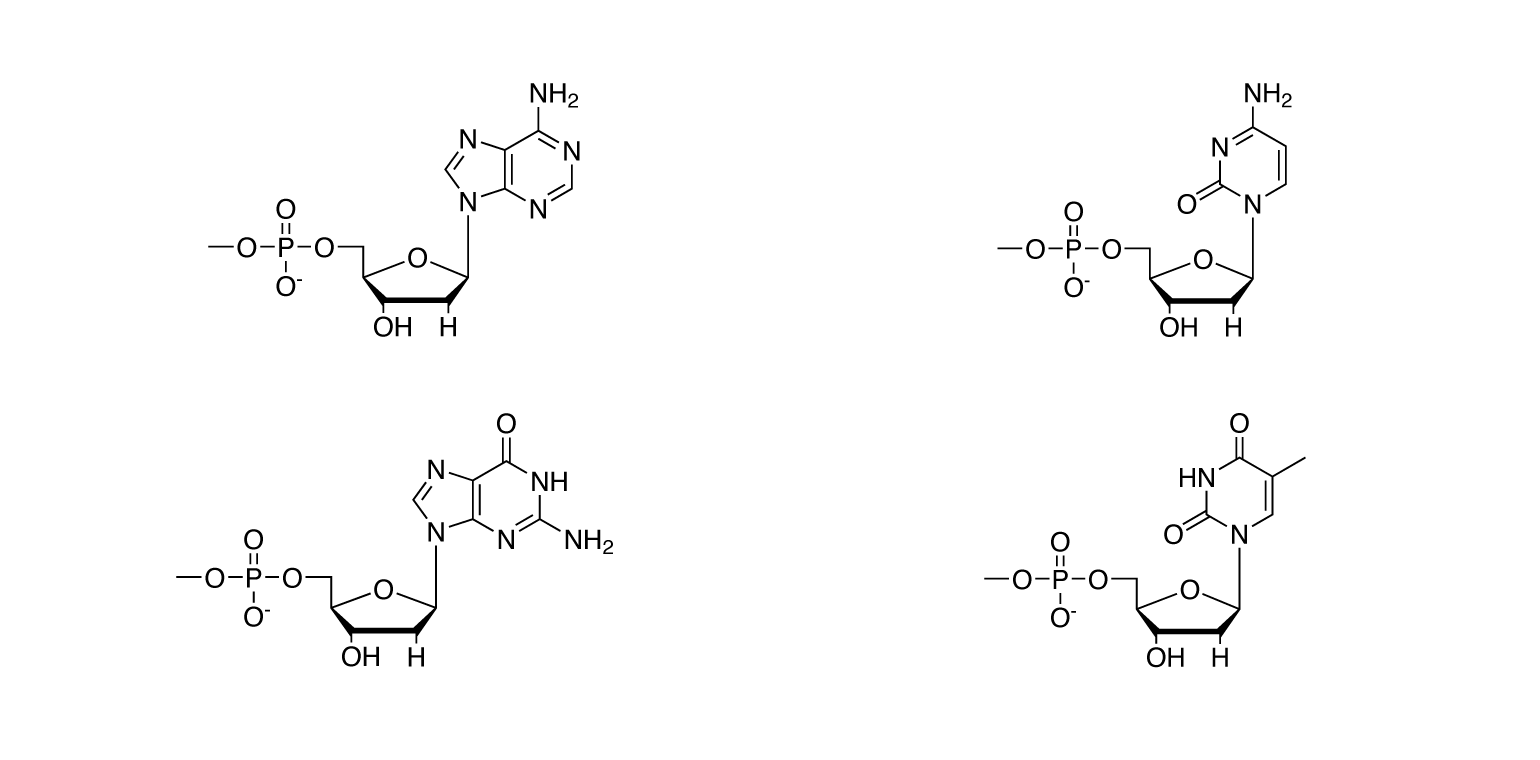
Deoxyribonucleotides (monophosphates, triphosphates)
Enzymes
We extract and purify enzymes and other functional ingredients from natural products such as animal tissues, using large freeze-drying equipment and other facilities.
-
N-Acetylneuraminic acid aldolase; N-Acetylneuraminic pyruvate lyase [ EC 4.1.3.3 ]
| Origin | Escherichia coli | Reaction | N-Acetylneuraminate → N-Acetyl-D-mannosamine + pyruvate |
|---|---|---|---|
| Appearance | White amorphous powder | Package | 10 units, Bulk |
- Origin
- Escherichia coli
- Reaction
- N-Acetylneuraminate → N-Acetyl-D-mannosamine + pyruvate
- Appearance
- White amorphous powder
- Package
- 10 units, Bulk
Properties
| Molecular weight | Approx. 98,000 Da (gel filtration) | ||
|---|---|---|---|
| Substrate specificity | N-glycolylneuraminic acid (NGNA) is cleaved as well as NANA. | ||
- Molecular weight
- Approx. 98,000 Da (gel filtration)
- Substrate specificity
- N-glycolylneuraminic acid (NGNA) is cleaved as well as NANA.
-
Neuraminidase [Sialidase]; Acylneuraminyl Hydrolase [ EC 3.2.1.18 ]
| Origin | Arthrobacter ureafaciens | Reaction | Sialyl compound → Sialic acid + Asialocompound |
|---|---|---|---|
| Appearance | White lyophilized powder | Package | 5 units, 100 units, Bulk |
- Origin
- Arthrobacter ureafaciens
- Reaction
- Sialyl compound → Sialic acid + Asialocompound
- Appearance
- White lyophilized powder
- Package
- 5 units, 100 units, Bulk
Properties
This enzyme consist of four isozymes (L, M1, M2, S).
| Molecular weight | Approx. 52,000 Da, 66,000 Da and 88,000 Da (gel filtration, SDS-PAGE) | ||
|---|---|---|---|
| Substrate specificity | The α-ketosidic linkage of N-glycolylneuraminic acid (NGNA) can be hydrolyzed as well as that of NANA. This enzyme cleaves α(2→3), α(2→6) and α(2→8) linkages of N-acetylneuraminic acid in glycoconjugates. The acivity is independent on Ca2+ and is not inhibited by EDTA, which is in striking contrast to Vibrio cholerae neuraminidase, and is not or slightly inhibited by inhibitors such as monoiodoacetate, p-chloromercuribenzoate and HgCl2, which is in striking contrast to Clostridium perfringens neuraminidase. | ||
- Molecular weight
- Approx. 52,000 Da, 66,000 Da and 88,000 Da (gel filtration, SDS-PAGE)
- Substrate specificity
- The α-ketosidic linkage of N-glycolylneuraminic acid (NGNA) can be hydrolyzed as well as that of NANA. This enzyme cleaves α(2→3), α(2→6) and α(2→8) linkages of N-acetylneuraminic acid in glycoconjugates. The acivity is independent on Ca2+ and is not inhibited by EDTA, which is in striking contrast to Vibrio cholerae neuraminidase, and is not or slightly inhibited by inhibitors such as monoiodoacetate, p-chloromercuribenzoate and HgCl2, which is in striking contrast to Clostridium perfringens neuraminidase.
-
Neuraminidase isozyme S [Reagent for studies of gangliosides]
| Origin | Arthrobacter ureafaciens | ||
|---|---|---|---|
| Appearance | White amorphous powder | Package | 1 unit, 5 units |
- Origin
- Arthrobacter ureafaciens
- Appearance
- White amorphous powder
- Package
- 1 unit, 5 units
Properties
| Molecular weight | Approx. 52,000 Da (gel filtration, SDS-PAGE) | ||
|---|---|---|---|
| Substrate specificity | sozyme S cleaves α(2→3), α(2→6) and α(2→8) linkages of N-acetylneuraminic acid in glycoconjugates. In the absence of detergents and calcium ion, the enzyme hydrolyzes N-acetylneuraminosyl moiety of polysialogangliosides and produces monosialoganglioside GM1, while in the presence of detergents (Na-cholate, Triton X-100 etc.), GM1 is further desialylated to asialoganglioside GA1. the character of the enzyme is similar to Vibrio cholerae neuraminidase except requirement of Ca2+ in the latter. | ||
- Molecular weight
- Approx. 52,000 Da (gel filtration, SDS-PAGE)
- Substrate specificity
- sozyme S cleaves α(2→3), α(2→6) and α(2→8) linkages of N-acetylneuraminic acid in glycoconjugates. In the absence of detergents and calcium ion, the enzyme hydrolyzes N-acetylneuraminosyl moiety of polysialogangliosides and produces monosialoganglioside GM1, while in the presence of detergents (Na-cholate, Triton X-100 etc.), GM1 is further desialylated to asialoganglioside GA1. the character of the enzyme is similar to Vibrio cholerae neuraminidase except requirement of Ca2+ in the latter.
-
3α-Hydroxysteroid dehydrogenase; 3α-Hydroxysteroid:NAD(P)+ oxidoreductase [ EC 1.1.1.50 ]
-
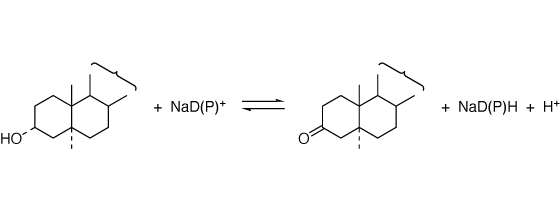
3α-Hydroxysteroid ⇄ 3-oxosteroid
| Origin | Pseudomonas testosteroni | ||
|---|---|---|---|
| Appearance | White lyophilized powder | Package | Bulk |
- Origin
- Pseudomonas testosteroni
- Appearance
- White lyophilized powder
- Package
- Bulk
Properties
| Molecular weight | Approx. 37,000 Da | ||
|---|---|---|---|
- Molecular weight
- Approx. 37,000 Da
-
β-Hydroxysteroid dehydrogenase; 3(or 17)β-Hydroxysteroid:NAD(P)+ oxidoreductase [ EC 1.1.1.51 ]
-
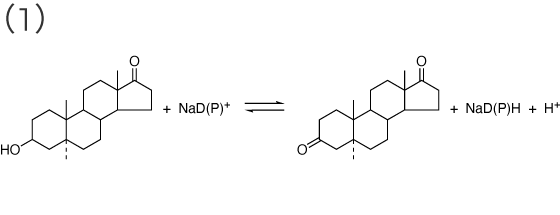
epi-androsterone ⇄ 5α-androstene-3,17-dione 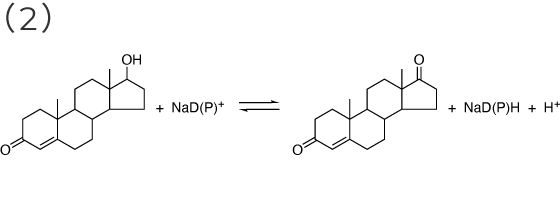
testosterone ⇄ 4-androstene-3,17-dione
| Origin | Pseudomonas testosteroni | ||
|---|---|---|---|
| Appearance | White amorphous powder | Package | 10 units |
- Origin
- Pseudomonas testosteroni
- Appearance
- White amorphous powder
- Package
- 10 units
Properties
| Molecular weight | Approx. 80,000 Da | ||
|---|---|---|---|
- Molecular weight
- Approx. 80,000 Da
-
NADH oxidase [ EC 1.6.3.1 ]
| Origin | Bacillus licheniformis | Reaction | NADH + H+ + O2 → NAD+ + H2O2 |
|---|---|---|---|
| Appearance | White lyophilized powder | Package | 100 units, Bulk |
- Origin
- Bacillus licheniformis
- Reaction
- NADH + H+ + O2 → NAD+ + H2O2
- Appearance
- White lyophilized powder
- Package
- 100 units, Bulk
Properties
| Molecular weight | Approx. 240,000 Da | ||
|---|---|---|---|
| Substrate specificity | In the absence of added FAD both NADH and NADPH are oxidized equally, but by the addition of FAD (about 30 μM) to reaction mixture the reaction velocity to NADH is accelerated about 20–30 times in contrast to 2–3 times of NADPH. Accordingly, the substrate specificity of NADH is about 10 times larger than that of NADPH in the presence of added FAD. | ||
- Molecular weight
- Approx. 240,000 Da
- Substrate specificity
- In the absence of added FAD both NADH and NADPH are oxidized equally, but by the addition of FAD (about 30 μM) to reaction mixture the reaction velocity to NADH is accelerated about 20–30 times in contrast to 2–3 times of NADPH. Accordingly, the substrate specificity of NADH is about 10 times larger than that of NADPH in the presence of added FAD.
-
Elastase [ EC 3.4.21.36 ]
Enzymatic API for improving lipid metabolism disorders.
Prepared by use of large freeze-drying equipment and other facilities.
-
Kalliginogenase [ EC 3.4.21.35 ]
Enzymatic API for improving circulatory disorders.
Prepared by use of large freeze-drying equipment and other facilities.
-
Phospholipase A2 [ EC 3.1.1.4 ]
For better quality of food with our original technology of extraction and purification
Phospholipase A2 is distributed widely in living organisms and well-known to digest phospholipids from plants and produce bioactive substances such as prostaglandine by a hydrolysis of phospholipids in the cell membrane. SANYO FINE has original technologies for extraction and purification of phospholipase A2 from swine pancreas. Lysonase is one of our products containing phspholipase A2 with high potency and its ability is stereo-selective hydrolysis of the ester at 2-position of glycero-phospholipids. We can supply them both as aqueous solution and powder form depending on our customer’s need.
Phospholipase A2 converts lecithin, which is contained in egg yolk and soybean, to lysolecithin by a hydrolysis. Lysolecithin has much ability of emulsification than that of lecithin and is used as emulsion stabilizer for ice-cream and mayonnaise, dispersion stabilizer for chocolate and quality improvement for dough.
-